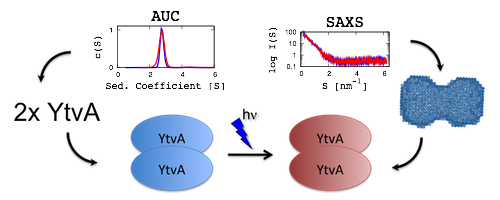
The switch that does not flip - the blue-light receptor YtvA from Bacillus subtilis adopts an elongated dimer conformation independent of the activation state as revealed by a combined AUC and SAXS study
M. Jurk; M. Dorn; A. Kikhney; D. Svergun; W. Gärtner; P. Schmieder
J. Mol. Biol. 403, 78-87 (2010)
Photoreceptors play an important role in plants and bacteria by converting extracellular stimuli into intracellular signals. One distinct class are the blue-light sensitive phototropins harbouring a light, oxygen, voltage (LOV) domain coupled to various effector domains. Photon-absorption by the chromophore within the LOV domain results in an activation of the output domain via mechanisms that are hitherto not well understood. The photoreceptor YtvA from Bacillus subtilis is a bacterial analog of phototropins, consists of a LOV and a sulfate transporter/anti-sigma-factor antagonist (STAS) domain and is involved in the response of the bacterium to environmental stress. We present here analytical ultracentrifugation studies and small-angle x-ray scattering experiments, showing that YtvA is a dimer. On the basis of these results, we present a low-resolution model of the dimer in the dark and the lit state of the protein. In addition, we show that YtvA does neither change its oligomerization state nor its overall shape upon light activation.
