Real-time tracking of phytochrome's orientational changes during Pr photoisomerization
Y. Yang; M. Linke; T. von Haimberger; J. Hahn; R.A. Matute; L.González; P. Schmieder; K. Heyne*
J. Am. Chem. Soc. 134, 1408-1411 (2012)
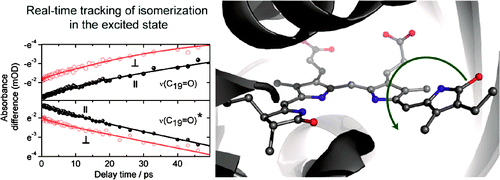
Photoisomerization of a protein bound chromophore is the basis of the light sensing and signaling responses of many photoreceptors. Z-to-E photoisomerization of the Pr Cph1?2 phytochrome has been investigated by polarization resolved femtosecond visible pump-infrared probe spectroscopy, which yields structural information on the Pr excited state (Pr*), Pr ground state, and lumi-R product state. By exhaustive search analysis two photoreaction time constants of (4.7±1.4) ps and (30±5) ps were found. Ring D orientational change in the electronic excited state to the transition state (90° twist) has been followed in real-time. Rotation of ring D takes place in the electronically excited state with a time constant of 30±5 ps. The photoisomerization is best explained by a single rotation around C15=C16 methine bridge in the Pr* state and diffusive interaction with its protein surrounding.
