A General One-Pot Synthesis of 2H-Indazoles Using an Organophosphorus-Silane System
J. Schöne; H. Bel Abed; P. Schmieder; M. Christmann; M. Nazaré *
Chemistry 24, 9090 - 9100 (2018)
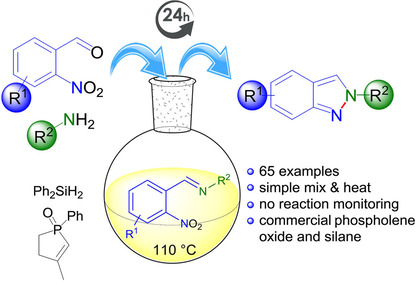
A simple and direct approach for the regioselective construction of the privileged 2H-indazole scaffold is described. The developed one-pot strategy employs a phospholene mediated N-N bond formation to access 2H-indazoles. The amount of organophosphorus reagent was minimized by recycling the phospholene oxide with organosilanes as reductant. Starting from functionalized 2-nitrobenzaldehydes and primary amines a mild reductive cyclisation, using commercially available phospholene oxide and silanes, delivered a wide variety of substituted 2H-indazoles in good to excellent yields.
