Chemically Induced Vinylphosphonothiolate Electrophiles for Thiol-Thiol Bioconjugations
A.L. Baumann; S. Schwagerus; K. Broi; K. Kemnitz-Hassanin; C.E. Stieger; N. Trieloff; P. Schmieder; C.P.R. Hackenberger*
J. Am. Chem. Soc. 142, 9544 - 9552 (2020)
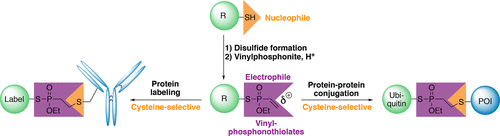
Herein we introduce vinylphosphonothiolates as a new class of cysteine-selective electrophiles for protein labeling and the formation of stable protein-protein conjugates. We developed a straightforward synthetic route to convert nucleophilic thiols into electrophilic, thiol-selective vinylphosphonothiolates: In this protocol, intermediately formed disulfides can be chemoselectively substituted with vinylphosphonites under acidic conditions to yield the desired vinylphosphonothiolates. Notably, this reaction sequence enables the installation of vinylphosphonothiolate electrophiles directly on cysteine side chains within peptides and proteins. In addition to labeling the monoclonal antibody trastuzumab with excellent cysteine-selectivity, we applied our protocol for the site-specific conjugation of two proteins with unique cysteine residues yielding a nonhydrolyzable phosphonothiolate-linked diubiquitin and an ubiquitin-α-synuclein conjugate. The latter was recognized as a substrate in a subsequent enzymatic ubiquitination reaction.
