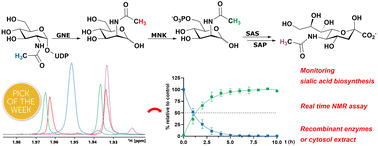
Real-time monitoring of the sialic acid biosynthesis pathway by NMR
J.L. Gorenflos López; P. Schmieder ; K. Kemnitz-Hassanin; H.C. Asikoglu; A. Celik; C.E. Stieger; D. Fiedler; S. Hinderlich; C.P.R. Hackenberger
Chem. Sci. 14, 3482-3492 (2023)
Sialic acids are part of the outermost component of the glycocalyx of all vertebrates; as such, they are fundamental markers in physiological and pathological processes. In this study, we introduce a real-time assay to monitor individual enzymatic steps of sialic acid biosynthesis, either with recombinant enzymes, in particular using UDP-N-acetylglucosamine 2-epimerase (GNE) or N-acetylmannosamine kinase (MNK), or in cytosolic rat liver extract. Using state-of-the-art NMR techniques, we are able to follow the characteristic signal of the N-acetyl methyl group, which displays different chemical shifts for the biosynthesis intermediates UDP-N-acetylglucosamine, N-acetylmannosamine (and its 6-phosphate) and N-acetylneuraminic acid (and its 9-phosphate). Pseudo 2- and 3-D NMR demonstrated that in rat liver cytosolic extract, the phosphorylation reaction of MNK is exclusive for N-acetylmannosamine generated by GNE. Thus, we speculate that phosphorylation of this sugar from other sources (e.g. external application to cells) or N-acetylmannosamine derivatives often applied in metabolic glycoengineering is not conducted by MNK but by a yet unknown sugar kinase. Competition experiments with the most prevalent neutral carbohydrates demonstrated that of these, only N-acetylglucosamine slowed N-acetylmannosamine phosphorylation kinetics, suggesting an N-acetylglucosamine-preferring kinase as the acting enzyme.
