NMR spectroscopic investigation of micropolymorphism-dependent dynamics of human major histocompatibility antigens |
 |
 |
 |
|
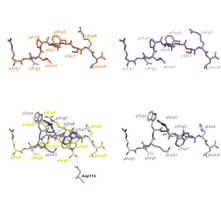
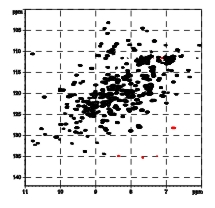
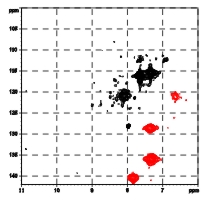
Antigens encoded by the major histocompatibility complex (MHC, HLA complex in human) play a central role in the immune response. MHC class I molecules accommodate small peptide fragments of 8 to 12 amino acids within a binding groove and present them to T cell receptors on cytotoxic T cells. T cells constitute a necessary component of normal adaptive immune responses, but can also be involved in autoimmunity. A wealth of structural information on MHC:peptide complexes has become available, but the static picture resulting from crystallographic studies has not been able to fully explain several features of the interaction between these molecules. We will employ heteronuclear NMR spectroscopy in conjunction with extensive labeling to investigate the dynamics of the peptide and the MHC class I binding groove at physiological temperature. By comparing peptides in complex with HLA-B27 subtypes that differ only by a single amino acid exchange from each other, but are differentially associated with an autoimmune disease, we will correlate the dynamic and structural attributes with distinct functional properties of these peptide-loaded molecules. |
|
Publications |
||
| C.S. Hee; M. Beerbaum; B. Loll; M. Ballaschk; P. Schmieder; B. Uchanska-Ziegler; A. Ziegler* ; "Dynamics of free versus complexed ß(2)-microglobulin and the evolution of interfaces in MHC class I molecules"; Immunogenetics 65, 157-172 (2013) |
last changes 01.03.2013, Peter Schmieder